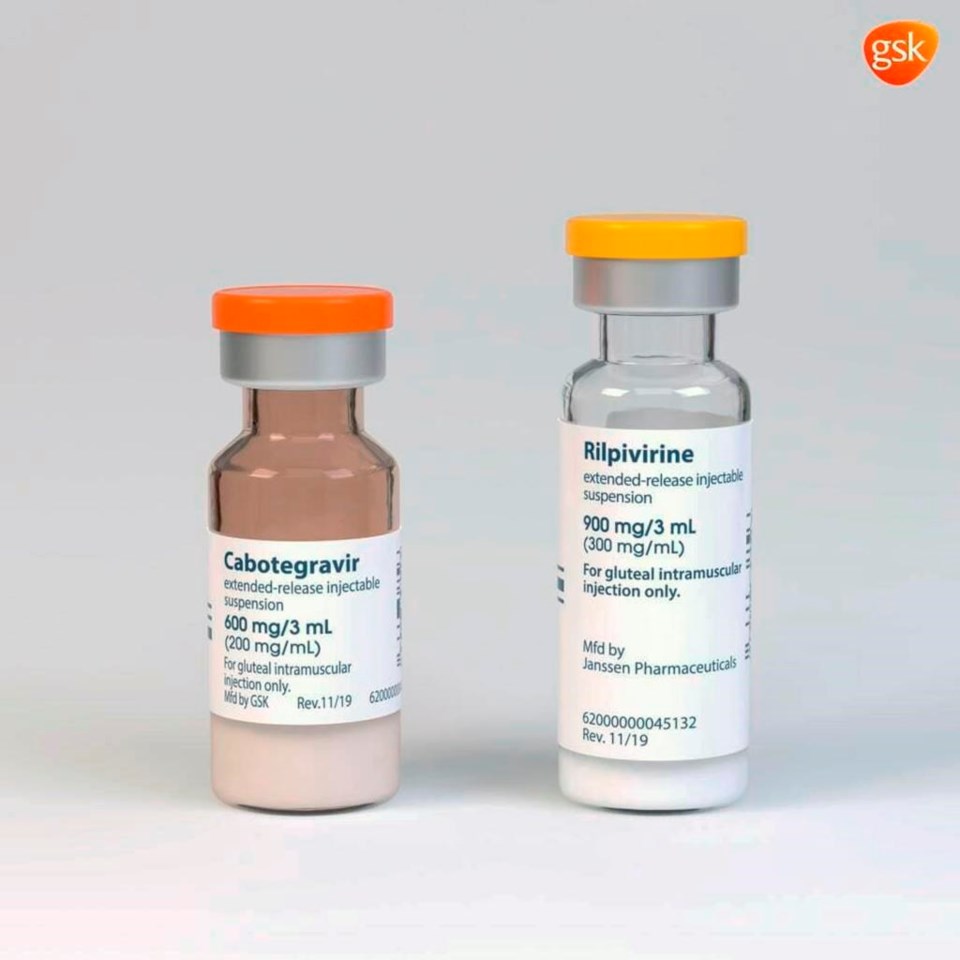
VANCOUVER — British Columbians with HIV have less access to an injectable drug compared to patients elsewhere in the country who can get a shot every two months instead of taking a daily pill, says an infectious diseases specialist.
Dr. Brian Conway questioned why the –°¿∂ ”∆µ Centre for Excellence in HIV/AIDS, and not the prescribing health practitioner, decides who qualifies for the extended release injectable drug called Cabenuva.
The centreprocures, distributes and monitors HIV medications in –°¿∂ ”∆µ, and also reviews applications from prescribers, unlike in other provinces without such a centralized system.
Conway said the centre declined approval of Cabenuva for all 15 patients he has applied for since the spring. Six of the applicants were rejected simply because they were deemed to be responding well to the drug in a pill form, he added.
"It's a long process to apply, which in my case has led to zero approvals," Conway said of the drug that he believes should be prescribed based on discussions between care providers and patients, without "unnecessary" oversight.
"Instead of it just being a regular prescription as it is for all the other HIV medications we have, it's a 25-question questionnaire and supporting documentation."
A spokesman for the centre said it has received 39 applications for Cabenuva since last year and 15 of them have been approved while 11 are still pending and 13 were withdrawn or have not been processed for other reasons.
Patients could be denied the injectable for various reasons, including not being able to tolerate one or both of the drugs it contains or having adverse reactions to them, the centre said.
Dr. Julio Montaner, the centre's executive director, was not available to provide details about the decision-making process.
Conway said 10 of his approximately 500 patients with HIV are taking Cabenuva because they got it in a clinical trial and continued takingitbefore Canada became the first country to approve the long-lasting treatment three years ago.
There are many benefits to the injectable versus a daily pill and patients should have the option without a third party making that decision, he said.
"Some people just don't want to be reminded every day that they have HIV. They're afraid people will find their pills," he said.
Cabenuva is a combination of the drugs cabotegravir and rilpivirine for patients aged 12 and older. Studies have shown it is as effective as oral therapy in treating thevirus that can be transmitted through sexual contact, by sharing drug-injection equipment and to children during pregnancy, delivery and breastfeeding.
The injectable HIV drug is considered a breakthrough in the evolution of medicines for patients with the virus who once had to take multiple daily pills, often with debilitating, visible side-effects such as loss of fat and muscle and sunken cheeks.
The –°¿∂ ”∆µ Centre for Excellence in HIV/AIDS spokesman said the organization has had an administrative role in dealing with HIV medications since the 1990s, based on a decision by the province, which provides funding.
He said in an email that "this is different from other jurisdictions across the country and elsewhere."
Conway said the centre has cited lack of staff to provide the injection at a doctor's office or clinic, which must also be equipped with a fridge to store the medication. However, many offices, including his own, have a fridge and administering the shot would not be different from many other types of injections, he added.
The –°¿∂ ”∆µ Health Ministry did not respond to questions about why patients do not have direct access to Cabenuva with a prescription from their doctor. It said the centre establishes guidelines for health-care providers to be involved in prescribing and monitoring of all antiretroviral drugs.
Sarah Chown, executive director of AIDS Vancouver, said members whose applications for Cabenuva have been rejected do not understand why that decision was made by an organization whose decision makers had not met them.
She said applicants who would rather get a shot every couple of months are required to provide a letter describing why they are unable to take a pill every day.
"We don't believe that patients should have to prove they are unable to continue oral medications in order to access the freedom and the privacy and the flexibility that comes with long-acting treatment," she said.
"We're curious and frustrated that here in –°¿∂ ”∆µ people are still getting rejected, especially because we know that some of those folks would be approved in other provinces."
The Ontario Ministry of Health said any HIV patient whose doctor has prescribed Cabenuva can get it at a pharmacy, the same as any other medication.
"There is no requirement to apply for case-by-case approval," it said. "As such, there is also no application process or denial."
Dr. Jonathan Angel, director of the HIV clinic at The Ottawa Hospital, said the prescribing of Cabenuva is an inexplicably "complex" issue for patients in –°¿∂ ”∆µ
"They're the ones that haven't had a loud enough or strong enough voice to get these drugs in –°¿∂ ”∆µ," he said.
Angel, who has prescribed Cabenuva to 127 patients since 2020, said some patients would rather continue taking a daily pill because they don't like injections but the choice should be theirs.
The cost of Cabenuva is believed to be similar to the most common daily HIV medication, called Biktarvy, so that should not be a factor in not approving it for patients in –°¿∂ ”∆µ, he said.
Individual provinces negotiate prices on some medications directly with drug companies, and those costs are confidential.
This report by The Canadian Press was first published Nov. 12, 2023.
Canadian Press health coverage receives support through a partnership with the Canadian Medical Association. CP is solely responsible for this content.
Camille Bains, The Canadian Press